Augmentation therapy is the only specific treatment for alpha1-antitrypsin (AAT) deficiency
Intravenous infusion of AAT, also known as augmentation therapy, is the only specific treatment available for AAT deficiency.1
Augmentation therapy has been demonstrated to raise levels of alpha1 antitrypsin in the blood and lungs above the threshold needed to protect lung tissue.2,3
- The goal of augmentation therapy is to correct the deficiency state and keep neutrophil elastase in check2,4
Serum levels of AAT during long-term therapy
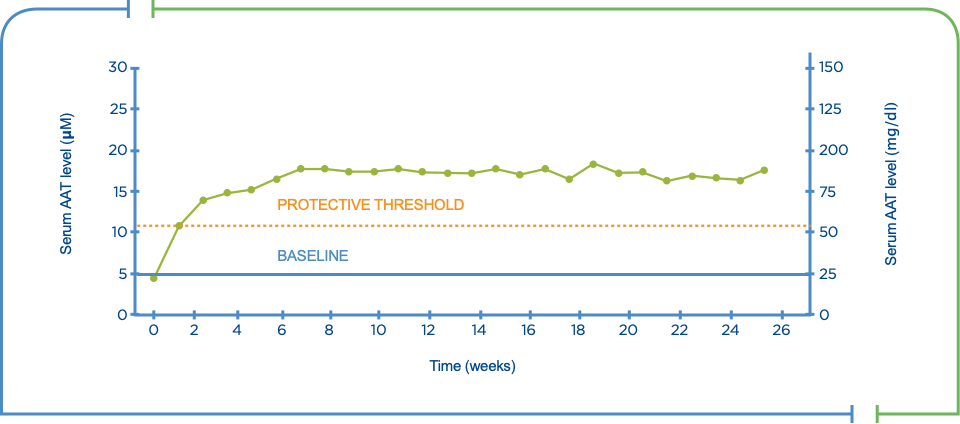
The American Thoracic Society/European Respiratory Society guidelines and the COPD Foundation Clinical Practice Guidelines recommend intravenous augmentation therapy for AAT deficiency*,3,5
The evidence for augmentation therapy
La cohorte de mayor tamaño y más larga de pacientes con déficit de AAT mostró que la supervivencia aumentó significativamente con el tratamiento sustitutivo.6
TASA DE MORTALIDAD ACUMULADA A 5 AÑOS PARA PACIENTES CON FEV1 INCIAL <50 % PREDICHO
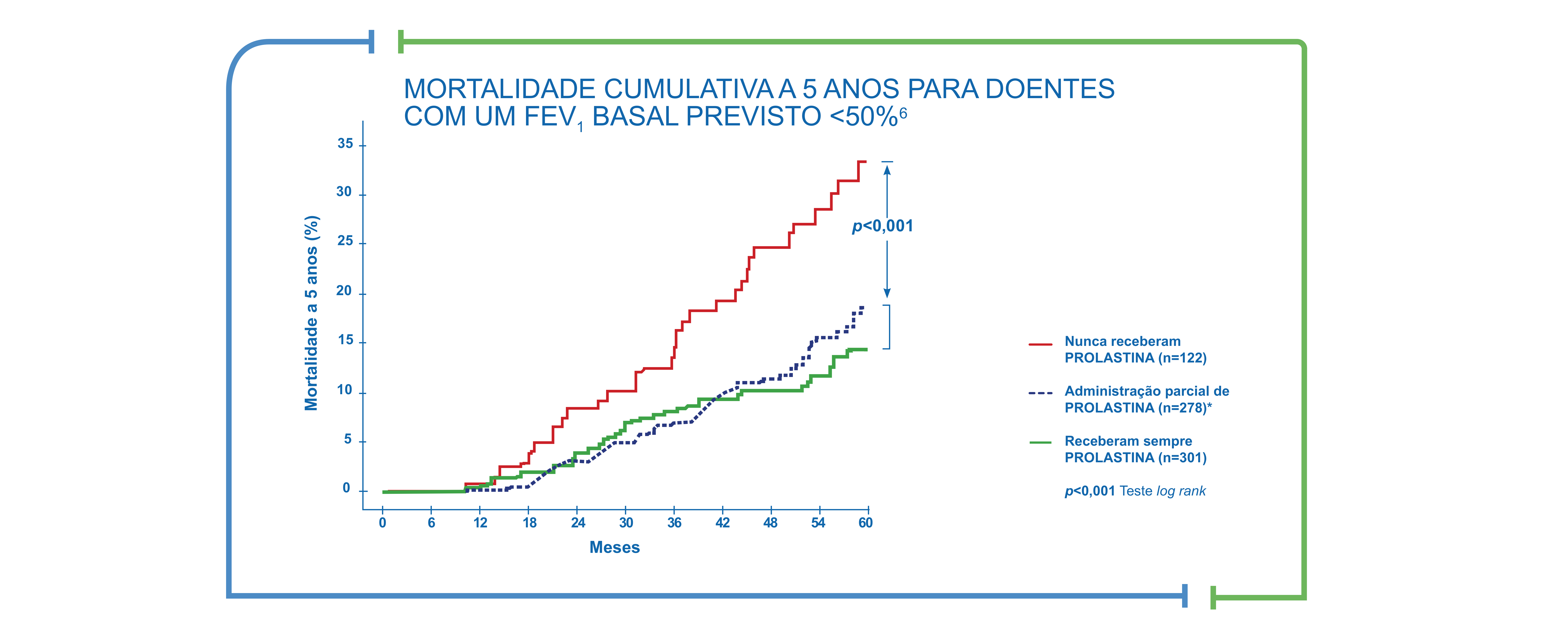
Los sujetos fueron inscritos en un registro del NHLBI y se incluyeron aquellos con niveles de AAT en suero inferiores a 11 μM (n=1026) o con el genotipo PiZZ (n=103). El objetivo era estudiar la disminución del FEV1 y la mortalidad en relación con la terapia de aumento y otros factores, incluido el consumo de tabaco. Los pacientes que recibían terapia de aumento parcial lo recibieron durante el 66% del período total; la mayoría de las interrupciones (59%) se atribuyeron a la recepción de un trasplante de pulmón.6
Augmentation therapy resulted in a significant reduction in the rate of emphysema progression7
ANÁLISIS INTEGRADO: PROGRESIÓN DEL ENFISEMA CON TERAPIA DE AUMENTO VS. PLACEBO
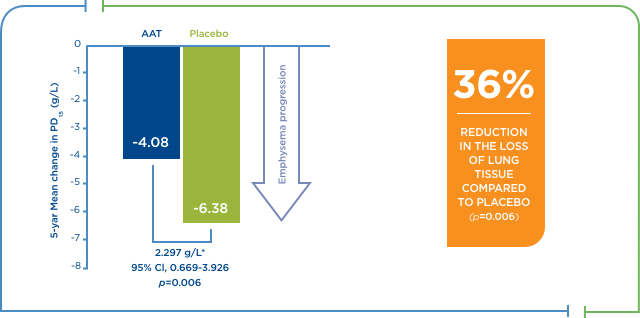
Resultados integrados de dos ensayos aleatorizados controlados con placebo de diseño y duración similares con 119 pacientes, 60 de los cuales fueron tratados con tratamiento sustitutivo. El estudio duró dos años y medio. El criterio de entrada del FEV1 para el primer estudio fue de un 30-80 % del previsto, y el tratamiento consistió en 250 mg/kg de peso corporal cada 4 semanas. En el segundo, el criterio de entrada del FEV1 fue de un 25-80 % del previsto, y el tratamiento consistió en 60 mg/kg de peso corporal semanalmente. La pérdida de tejido pulmonar y el efecto del tratamiento sustitutivo en sujetos alfa-1 se midió con una densitometría por TAC.
La terapia de aumento tuvo como resultado una reducción significativa de la tasa de progresión del enfisema.7
AAT: alpha1 antitrypsin; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CT: computed tomography; FEV1: forced expiratory volume in 1 second; NHLBI: National Heart, Lung and Blood Institute; PD15: change in 15th percentile of lung density.
*Recommended for individuals with FEV1 35-60% of predicted.
References
- Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. European Respiratory Journal. 2017;50(5).
- Wewers MD, Casolaro MA, Sellers SE, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055-62.
- ATS, ERS. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818-900.
- Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365(9478):2225-36.
- Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult (Clinical Practice Guidelines). Chronic Obstr Pulm Dis (Miami). 2016;3(3):668-82.
- The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49-59.
- Stockley RA, Parr DG, Piitulainen E, et al. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136.



A short-term study was performed to evaluate safety and dosing of alpha1 antitrypsin to maintain serum levels above 80 mg/dL. 21 patients with the PiZZ genotype were given 60 mg of active, plasma-derived, alpha1 antitrypsin per kg of body weight for 6 months.